
(John Dalton)
The concept on Atomic Theory on a firm scientific basis was first proposed by a British School Teacher, John Dalton in 1808 which was regarded as Dalton’s Atomic Theory.
According to Dalton’ Atomic Theory,
- Matter consists of indivisible particle called atoms.
- All atoms of a given element have identical mass and identical properties. Atoms of different elements differ in mass.
- Compounds are formed when atoms of different elements combine in a fixed ratio.
- Chemical reactions involve by reorganization of atoms. These are neither created nor destroyed.
Though this atomic theory could explain many laws such as law of conservation of mass, Law of constant proportion and the law of multiple proportion ,but failed to explain the indivisibility of atoms by gradual research and development by different scientists atoms during last part nineteenth and early first part of twentieth century with a conclusion that atom can be further divided into sub-atomic particles, such as electrons, protons and neutrons. Many more challenges were met with the scientists regarding stability of atoms, chemical combinations and atomic emission /absorption of energy by electromagnetic radiation.
DISCOVERY OF ELECTRON:
In the middle part of nineteenth century, Faraday experimented with electric discharge through Cathode Ray Discharge Tube. It consists of a glass tube with two thin pieces of metal sealed in it called electrodes. The electric discharge in the discharge tube was carried out in the partially evacuated gas or low pressure in cathode ray tube with application of high voltages across its electrodes. The electric discharge constitutes a stream of particles moving from cathode to anode. These were called Cathode Rays.
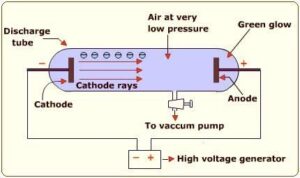
Observation from Cathode Ray Tube Experiment:
- Cathode rays starts from cathode and move towards the anode.
- The rays are themselves are not visible, but cause luminosity when striking against phosphorescent or fluorescent material.
- The rays travel in straight line in the absence of electric and magnetic field
- The behaviour of cathode rays in the presence of electric or magnetic field is same as in case of negatively charged particle. It suggests that the cathode rays consists of negatively charged particles, called electrons.
- The characteristics of cathode rays do not depend upon material of electrode and the nature of gas present in cathode ray tube.
CONCLUSION: The electrons are basic constituent of all the atoms.
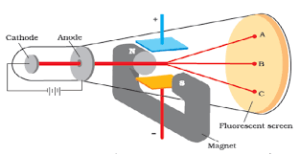
CHARGE TO MASS RATIO OF ELECTRON: In 1897,British Physicist, J J Thomson measured the electric charge to mass of the electron ( e/m) by using cathode ray tube by application of electric and magnetic field perpendicular to each other in the path of the electron.It was found to be 1.758820x Coul/Kg
CHARGE ON THE ELECTRON: The charge of the electron was determined by another scientist, R A Millikan by oil drop experiment in 1906-14.which was found to be 1.6x 10C which very close to present accepted value 1.6022x 10-19C. Consequently, mass of the electron(e) was found to be 9.1094Kg
DISCOVERY OF PROTONS & NEUTRONS:
Electric discharge through modified cathode ray tube(perforated cathode) led to discovery of stream of positively charged material particles at the back side of perforated cathode which was known as canal rays or positive rays. These positively charged material particles are protons. Later on, another Scientist Chadwick by bombardment of particles against thin beryllium sheet and discovered another electrically neutral particles called neutron whose mass is slightly greater than that of proton.
The mass of proton and neutron were found to be 1.67262x Kg and 1.67493x Kg respectively.
ATOMIC MODEL OF ATOM:
Different Atomic models were proposed to explain the structure atom and distribution of charged particles.
- Thomson Model of Atom:
This atomic model was proposed by J J Thomson in 1898.In his atomic model he proposed
- The atom to be spherical in shape with approximate diameter of m
- The positive charge is uniformly distributed through the sphere.
- The electrons are embedded into the sphere to give most stable electrostatic arrangement.
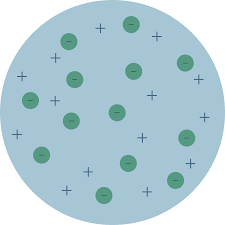
This atomic model known as Thomson’s Plum Pudding Model as the electrons are embedded as plums in a pudding as shown in figure.
RUTHERFORD’S NUCEAR MODEL OF ATOM:
Rutherford carried out his famous experiment by Bombardment of particles against a very thin gold foil. This was known as particle Scattering Experiment.
Description of Experiment: A stream of high energy particles from a radioactive source was directed at thin gold foil of thickness 100nm.The gold foil is surrounded by a fluorescent Zinc Sulphide Screen as shown in figure.
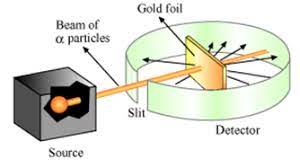
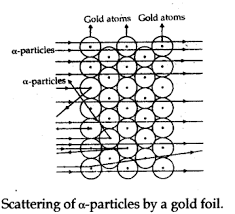
The particles produced luminosity striking the fluorescent screen.
Observation:
- Most of the particles passed through the gold foil undeflected.
- A small fraction of particles was deflected by small angles.
- A very few particles(one in 20000) bounced back.
CONCLUSION:
- Most of the space in the atom is empty as most of the particles passed through the foil undeflected.
- The positive charge is concentrated in a small volume at the central part of the atom as few nos of particles repelled and deflected.
- The volume occupied by the nucleus is negligibly small as compare to the total volume of the atom. The radius of the atom is about m compared that of metre
On the basis of above observation and conclusion, Rutherford proposed his nuclear model of atom as follows,
- The positive charge and most of the mass of the atom is densely concentrated at the central part in an extremely small region. This very small portion of the atom is called nucleus.
- The nucleus is surrounded by electrons moving around it in high speed in circular paths called orbits. Each atom is a miniature of solar system.
- Electrons and nucleus are held together by electrostatic forces of attraction.
DRAWBACKS OF RUTHERFORD ATOMIC MODEL:
At the time of Rutherford, another scientist, Clark Maxwell deduced law which is known as the law of electrodymics, which states that a charged whenever accelerated it emits radiation. As per Rutherford atomic model, an electron revolving in circular orbit subjected to acceleration, so it would emit radiation losing energy, follow a path a spiral and at last would fall to the nucleus, thus nuclear model of atom would collapse. But this does not happen. This could not be satisfactorily explained.
Further, Rutherford model of atom could not explain the electronic structure of the atom and energy of the electron.
SOME BASIC TERMS:
Atomic Number(Z):The atomic number of an element is the number of protons present in the nucleus of the atom of the element.
Mass Number (A):The mass number of an element is the sum of number of protons and number of neutrons(n) present inside the nucleus of the atom of the element.
A=Z+n
Isotopes: Atoms having same atomic number and different mass numbers are called isotopes e,g Hydrogen has three isotopes Protium( ),Deutorium( ) and Tritium( ).The isotope of an element are available in nature in fixed proportion. e.g protium is available in 99.985% in nature.
Isobars: Atoms having same mass number but different atomic numbers are known as isobars
The chemical properties of the atom is controlled by number of electrons present in the neutral atom. Hence , isotopes of the element show same chemical properties.
ELECTROMANGETIC RADIATION: James Maxwell (1870)first explained facts regarding electromagnetic radiations.Whenever a charged particle is accelerated, it produces alternating electric and magnetic field at right angle to each other transmitted in space in the form of wave. This is known as electromagnetic wave or electromagnetic radiation.
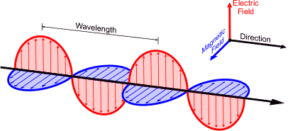
(Electromagnetic Radiation)
The electromagnetic radiation behaves as wave. Unlike other waves, it requires no material medium. The electromagnetic wave are of different types depending the value of their wavelengths (distance covered in one complete cycle)/frequencies(number of cycles per second)which constitute an electromagnetic spectrum. In velocity of electromagnetic radiation, c= , where c is constant for a particular medium.
Different regions of the electromagnetic spectrum are identified by different names such radio frequency region (around Hz) used for broadcasting purpose, microwave region ( Hz) used in radar, Infrared region( Hz) used for heating purpose and ultraviolet region. The electromagnetic radiations detected by human eye is visual region (380nm-700nm) .The red colour lies in lower frequency.
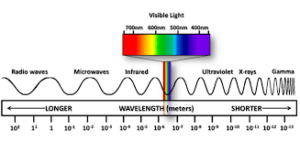
(Range of electromagnetic radiation)
region.where as blue colour lies between higher frequency region. The interference and diffraction are the properties of light which shows wave character of light
Black Body: It is an ideal body which emits or absorbs all frequencies of electromagnetic radiations.
